Vsepr Theory
Vsepr Theory
History[edit]
The idea of a correlation between molecular geometry and number of valence electron pairs (both shared and unshared pairs) was originally proposed in 1939 by Ryutaro Tsuchida in Japan,[7] and was independently presented in a Bakerian Lecture in 1940 by Nevil Sidgwick and Herbert Powell of the University of Oxford.[8] In 1957, Ronald Gillespie and Ronald Sydney Nyholm of University College London refined this concept into a more detailed theory, capable of choosing between various alternative geometries.[9][10]
Overview[edit]
VSEPR theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. A central atom is defined in this theory as an atom which is bonded to two or more other atoms, while a terminal atom is bonded to only one other atom.[1]: 398 For example in the molecule methyl isocyanate (H3C-N=C=O), the two carbons and one nitrogen are central atoms, and the three hydrogens and one oxygen are terminal atoms.[1]: 416 The geometry of the central atoms and their non-bonding electron pairs in turn determine the geometry of the larger whole molecule.
The number of electron pairs in the valence shell of a central atom is determined after drawing the Lewis structure of the molecule, and expanding it to show all bonding groups and lone pairs of electrons.[1]: 410–417 In VSEPR theory, a double bond or triple bond is treated as a single bonding group.[1] The sum of the number of atoms bonded to a central atom and the number of lone pairs formed by its nonbonding valence electrons is known as the central atom's steric number.
The electron pairs (or groups if multiple bonds are present) are assumed to lie on the surface of a sphere centered on the central atom and tend to occupy positions that minimize their mutual repulsions by maximizing the distance between them.[1]: 410–417 [11] The number of electron pairs (or groups), therefore, determines the overall geometry that they will adopt. For example, when there are two electron pairs surrounding the central atom, their mutual repulsion is minimal when they lie at opposite poles of the sphere. Therefore, the central atom is predicted to adopt a linear geometry. If there are 3 electron pairs surrounding the central atom, their repulsion is minimized by placing them at the vertices of an equilateral triangle centered on the atom. Therefore, the predicted geometry is trigonal. Likewise, for 4 electron pairs, the optimal arrangement is tetrahedral.[1]: 410–417
As a tool in predicting the geometry adopted with a given number of electron pairs, an often used physical demonstration of the principle of minimal electron pair repulsion utilizes inflated balloons. Through handling, balloons acquire a slight surface electrostatic charge that results in the adoption of roughly the same geometries when they are tied together at their stems as the corresponding number of electron pairs. For example, five balloons tied together adopt the trigonal bipyramidal geometry, just as do the five bonding pairs of a PCl5 molecule.
Steric number[edit]
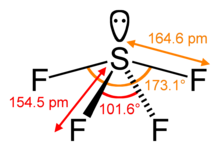
The steric number of a central atom in a molecule is the number of atoms bonded to that central atom, called its coordination number, plus the number of lone pairs of valence electrons on the central atom.[12] In the molecule SF4, for example, the central sulfur atom has four ligands; the coordination number of sulfur is four. In addition to the four ligands, sulfur also has one lone pair in this molecule. Thus, the steric number is 4 + 1 = 5.
Degree of repulsion[edit]
The overall geometry is further refined by distinguishing between bonding and nonbonding electron pairs. The bonding electron pair shared in a sigma bond with an adjacent atom lies further from the central atom than a nonbonding (lone) pair of that atom, which is held close to its positively charged nucleus. VSEPR theory therefore views repulsion by the lone pair to be greater than the repulsion by a bonding pair. As such, when a molecule has 2 interactions with different degrees of repulsion, VSEPR theory predicts the structure where lone pairs occupy positions that allow them to experience less repulsion. Lone pair–lone pair (lp–lp) repulsions are considered stronger than lone pair–bonding pair (lp–bp) repulsions, which in turn are considered stronger than bonding pair–bonding pair (bp–bp) repulsions, distinctions that then guide decisions about overall geometry when 2 or more non-equivalent positions are possible.[1]: 410–417 For instance, when 5 valence electron pairs surround a central atom, they adopt a trigonal bipyramidal molecular geometry with two collinear axial positions and three equatorial positions. An electron pair in an axial position has three close equatorial neighbors only 90° away and a fourth much farther at 180°, while an equatorial electron pair has only two adjacent pairs at 90° and two at 120°. The repulsion from the close neighbors at 90° is more important, so that the axial positions experience more repulsion than the equatorial positions; hence, when there are lone pairs, they tend to occupy equatorial positions as shown in the diagrams of the next section for steric number five.[11]
The difference between lone pairs and bonding pairs may also be used to rationalize deviations from idealized geometries. For example, the H2O molecule has four electron pairs in its valence shell: two lone pairs and two bond pairs. The four electron pairs are spread so as to point roughly towards the apices of a tetrahedron. However, the bond angle between the two O–H bonds is only 104.5°, rather than the 109.5° of a regular tetrahedron, because the two lone pairs (whose density or probability envelopes lie closer to the oxygen nucleus) exert a greater mutual repulsion than the two bond pairs.[1]: 410–417 [11]
A bond of higher bond order also exerts greater repulsion since the pi bond electrons contribute.[11] For example in isobutylene, (H3C)2C=CH2, the H3C−C=C angle (124°) is larger than the H3C−C−CH3 angle (111.5°). However, in the carbonate ion, CO2−
3, all three C−O bonds are equivalent with angles of 120° due to resonance.